What are actinocoresTM?
ActinocoresTM are the molecular cores of Actinomycete natural products. ActinocoresTM can be used at university labs, biotech startups and large companies to accelerate drug discovery research. Start with biologically inactive actinocoresTM and build them into biologically active pharmaceutical agents.

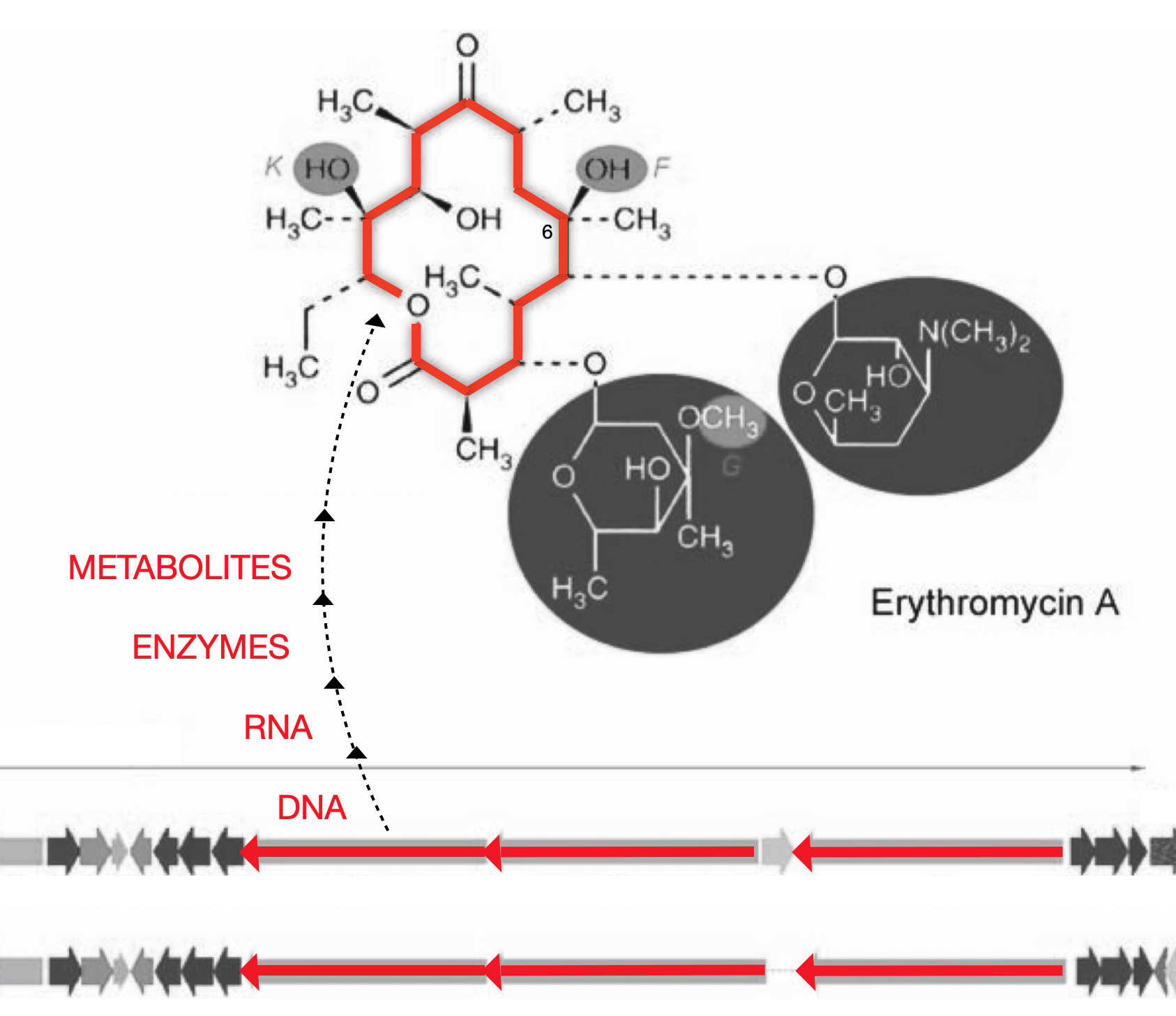
Figure 1. From DNA to ActinocoreTM
Actinomycete DNA (orange arrows) is at the center of the antibiotic gene cluster and is surrounded by tailoring genes (small gray arrows) that have been inactivated by a mutation created by Fermalogic. This core DNA codes for the erythronolide B (EB) actinocoreTM.
The Fermalogic mutation keeps the actinocoreTM (orange ring) from developing during fermentation into the fully developed natural product (orange ring + gray highlights). Instead, just the actinocoreTM is made. Drug discovery scientists can add tailoring groups to the actinocoreTM through chemical or biochemical means to create a final structure with the desired bioactivity.
What makes ActinocoresTM special?
Shaped by Nature
Unlike randomly generated chemically synthesized molecular scaffolds, evolution has shaped actinocoresTM through millions of years of natural selection to accept tailoring groups that lead to a multitude of bioactive products.
Plentiful types
ActinocoresTM already exist at the core of many approved drug products including macrolides from erythromycin to rapamycin, anthracyclines and beta lactams.
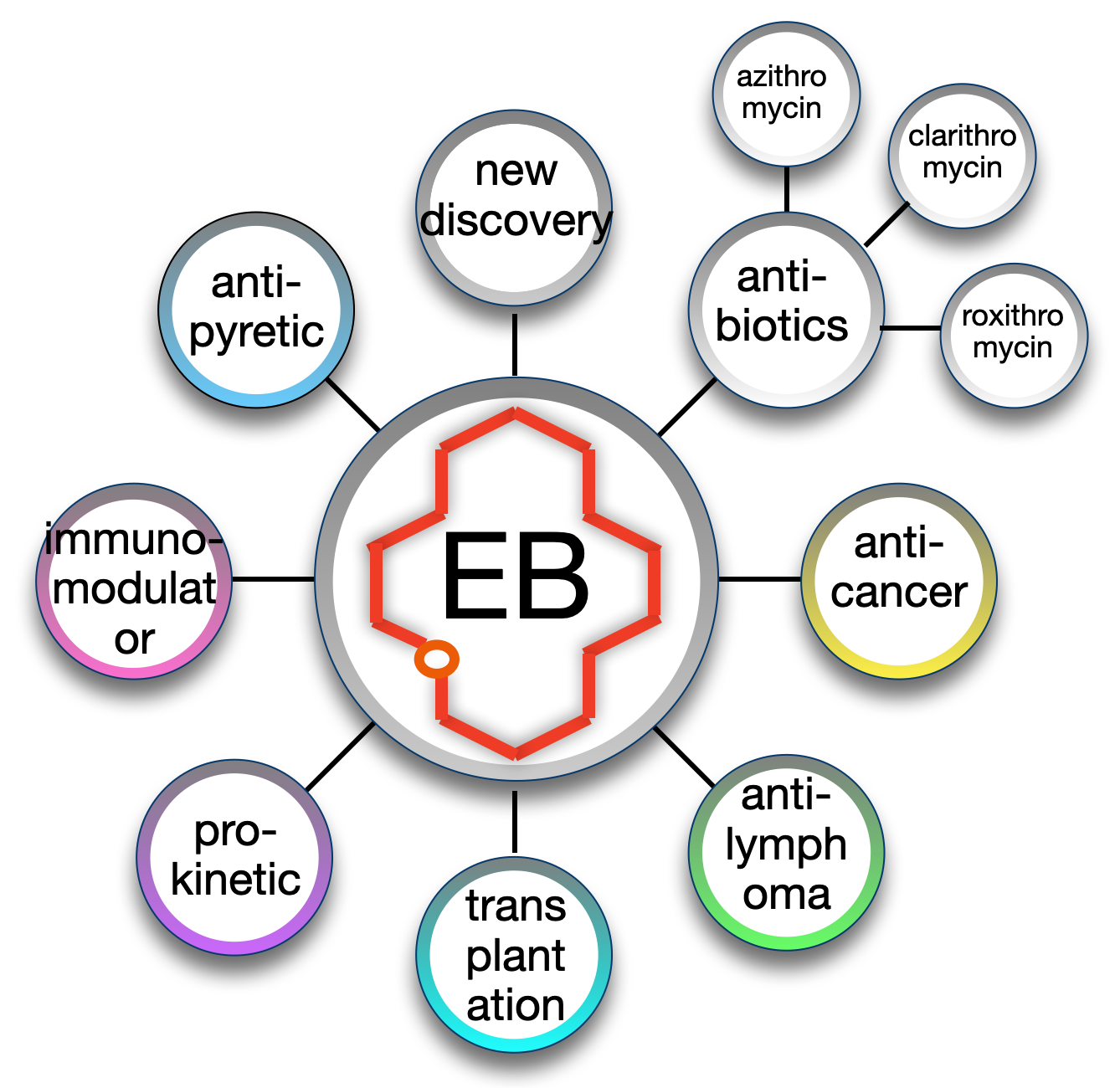
Versatility (Figure below)
Different classes of drugs can be developed from the same actinocoreTM. The macrolide actinocoreTM erythronolide B (EB) can be found at the core of at least eight different clinical or pre-clinical product categories including anti-cancer (1,2,3), immunomodulator (4), antipyretic (5), pro-kinetic (6), anti-inflamatory (7), transplantation (8) and antibiotic (9, see figure).

Example of a new antibiotic made with the EB actinocoreTM
Spagnoli et al., started with erythronolide B (EB) and made a new patentable antibiotic.
- Obtain an antibiotic producing actinomycete strain.
- Mutate the strain so that it no longer produces its native antibiotic.
- Cultivate the mutant strain in the presence of EB.
- Screen the fermentation extract for novel antibiotics.
Spagnoli et al., published this method in 1983 to create one of the first "hybrid" macrolide antibiotics. In this case a hybrid between erythromycin and oleandomycin. The challenge today is to see how powerful this process can be. Biochemical reactions will occur in unpredictable ways when EB is cultivated with other actinomycetes.


Erythronolide B (EB) ActinocoreTM
Roberto Spagnoli, Leonardo Cappelletti, and Luciano Toscano. Biological conversion of erythronolide B, an intermediate of erythromycin biogenesis, into new "hybrid" macrolide antibiotics. Journal of Antibiotics 36: 365-375, 1983 (PDF)
From Spagnoli et al.: "In recent years there has been a considerable interest in the search of biological routes to develop new antibiotics. Since a number of macrolide aglycones has been isolated, these compounds were found to be useful not only for the elucidation of biosyntheticpathways, but also as a temptative starting material (either as such or chemically modified) to obtain new molecules with antibiotic activity. Because of the structural analogies between the two most important 14-membered macrolides, erythromycin and oleandomycin, which have been suggested to reflect closely related biosynthetic pathways we assumed that new "hybrid" structures could be obtained by adding erythronolide B, an intermediate of erythromycin biogenesis in the fermentation medium of Streptomyces antibioticus ATCC 31771, a blocked mutant of an oleandomycin producing strain. Four new antibiotics I, II, III, IV (Scheme1) were successfully derived by such an approach. In this paper the preparation, structure determination and properties of these compounds are described."